-
WHAT IS NASA PHYSICS?
-
MODULES
-
Forces and Motion
-
Conservation of Momentum & Energy
-
Temperature and Heat
-
Fluids
-
Optics
-
Electromagnetic Spectrum
-
Modern Physics
-
Anticipation Guide 7
-
Intro to Modern Physics
-
Blackbody Radiation
-
The Ultraviolet Catastrophe
-
The Photoelectric Effect
-
Bohr's Atom
-
Spectra
-
Radioactive Decay
-
Special Relativity (SR)
-
Simultaneity
-
Distance and Time
-
General Relativity
-
May the Forces be with You
-
Modern Physics Notebook
-
Assessment Problems 7
-
-
Useful Things
-
-
SITE MAP
Temperature and Heat
-
Anticipation Guide
-
Thermal Energy
-
Measuring Temperature
-
Heat Expansion
-
Heat
-
Heat Capacity
-
Change of State
-
Transferring Heat
-
Greenhouse Effect
-
Notebook
-
Assessment Problems
3 ways to lose heat
conduction – touch
convection – mass movement
radiation – energy movement
themal conductivity, k, = calories per centimeter per second per degree C
Equation
H=(k * t * A * T)——————— d
3.9
Transferring Heat
There may be 50 ways to leave a lover, but there are only three ways to lose heat: conduction, convection and radiation. You have experienced all of these, sometimes painfully.
Pancakes; Image from The Sugar Muse Conduction is the way heat is transferred by touch. A hot skillet cooking a pancake transfers energy from the gas stove flames to the iron skillet to the initially cold pancake batter with embedded blueberries. Sometimes the handle of the skillet also conducts heat to the cook’s hand, causing the skillet to drop to the floor and cold cereal to be eaten for breakfast.
Conduction is the way heat is transferred by touch. A hot skillet cooking a pancake transfers energy from the gas stove flames to the iron skillet to the initially cold pancake batter with embedded blueberries. Sometimes the handle of the skillet also conducts heat to the cook’s hand, causing the skillet to drop to the floor and cold cereal to be eaten for breakfast.
Convection transfers heat by moving hot material, almost always upwards because hot stuff rises. For a breakfast on a cold morning, oats boil in an aluminum pot with a wooden handle. The flames warm the bottom of the pot by conduction and the bottom conducts heat into the oats/water mixture resting on the bottom of the pot. As the porridge and water warm they begin to move upwards, convecting heat to the top of the pan, and bubbles of steam break the surface carrying heat into the kitchen. A chunk of butter is added just to see the amorphous material slowly transform to liquid. Because of its wooden handle the pot is safely moved to the table and everyone enjoys a warm breakfast.
On a vastly larger scale, convection transports warm rocks from deep in the Earth’s mantle through the crust, where lava may erupt onto the surface. The lava then cools by radiation and convection, and even by conduction into the ground it flows over. Convection also occurs within stars, carrying huge amounts of heat to star’s surface where is radiated into space.
 Radiation transfers heat through the air (or a vacuum), not by moving material but by electromagnetic radiation. That is how energy from the Sun is transferred to Earth. The Sun’s surface is very hot – about 5,500ºC – and it radiates at various wavelengths including the infrared, which we feel as pleasant heat on a spring day. Radiation also includes wavelengths of energy corresponding to light, so we can see, and ultraviolet, which produces a pleasing copper glow to our skin or after a long day at the beach, sunburn. More prosaically, infrared radiation from glowing hot wires inside the slots of a toaster dry and singe bread to make a crisp brown surface texture to hold and melt butter. With a nice nice glass of orange juice from the fridge (chilled by convection that removed heat from originally warm juice), a thermally diverse breakfast can be enjoyed.
Radiation transfers heat through the air (or a vacuum), not by moving material but by electromagnetic radiation. That is how energy from the Sun is transferred to Earth. The Sun’s surface is very hot – about 5,500ºC – and it radiates at various wavelengths including the infrared, which we feel as pleasant heat on a spring day. Radiation also includes wavelengths of energy corresponding to light, so we can see, and ultraviolet, which produces a pleasing copper glow to our skin or after a long day at the beach, sunburn. More prosaically, infrared radiation from glowing hot wires inside the slots of a toaster dry and singe bread to make a crisp brown surface texture to hold and melt butter. With a nice nice glass of orange juice from the fridge (chilled by convection that removed heat from originally warm juice), a thermally diverse breakfast can be enjoyed.
Physics is more useful than just for cooking breakfast, and conduction, convection and radiation do many more important things. Heat is the movement of vibrational energy from cooler to warmer areas. In conduction, the molecules in a skillet directly touched by hot flames vibrate more quickly and their vibrational movement causes them to collide and transfer energy to nearby slower molecules. This continues all along the skillet, cooking the food or burning your hand.
Some materials are better at conducting heat than others. Metals are good conductors of heat and electricity, both being movement of electrons. Wood and glass and other materials with less regular molecular structure are poorer transmitters of energy and are often used as insulators. For example, a wood handle for a cooking pan, or glass insulators carrying high voltage wires on telephone poles.
The term thermal conductivity, with the symbol k, is a measure of how well a material conducts heat. The units for k are calories per centimeter per second per degree C, or more economically, cal/cm s °C.
 This listing shows why some pots and pans are made of aluminum but have their bottoms coated with copper. Copper conducts heat nearly twice as efficiently as aluminum, allowing pans to heat faster and to more evenly spread heat across the pan. Silver would be a little better but vastly more costly! With a k = 0.16 you can understand that iron skillets are very slow to heat up – and to cool. Nonetheless, many cooks prefer them because they slowly build up a very even heat, perfect for stews that require a long time to cook. Iron skillets can maintain very high cooking temperatures so they are also excellent for frying food. And they are more effective to bang against the heads of burglars than light aluminum pans.
This listing shows why some pots and pans are made of aluminum but have their bottoms coated with copper. Copper conducts heat nearly twice as efficiently as aluminum, allowing pans to heat faster and to more evenly spread heat across the pan. Silver would be a little better but vastly more costly! With a k = 0.16 you can understand that iron skillets are very slow to heat up – and to cool. Nonetheless, many cooks prefer them because they slowly build up a very even heat, perfect for stews that require a long time to cook. Iron skillets can maintain very high cooking temperatures so they are also excellent for frying food. And they are more effective to bang against the heads of burglars than light aluminum pans.
Physicists calculate the amount of heat that flows by conduction with this formula:
Conductive heat = (thermal conductivity x time x area x temperature) / thicknessH = (k * t * A * T)——————— d
Here is an example. How much heat flows through a glass window in winter in one hour?
Here are the meanings of the terms in the equation and their values for this example:
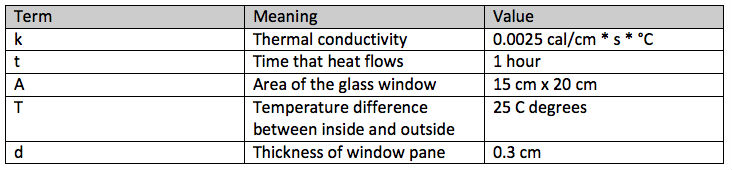
With these values H = heat loss in calories =
(0.0025 cal/(cm * s * °C) * 60 min * 60 s/min * 15 cm * 20 cm * 25 °C) / 0.3cm = 225,000 calories
(notice how all the units cancel out, leaving only calories)
 This is a lot of energy, which is why we run our furnaces so often in the winter, replacing the continuing loss. Many energy-conscious home owners use windows with two panes of glass with the narrow space between filled with very low conductivity gas such as argon. These windows greatly cut down heat loss and thus save money because less heating is needed.
This is a lot of energy, which is why we run our furnaces so often in the winter, replacing the continuing loss. Many energy-conscious home owners use windows with two panes of glass with the narrow space between filled with very low conductivity gas such as argon. These windows greatly cut down heat loss and thus save money because less heating is needed.
The designers of Space Shuttle windows were very concerned about heat transfer too. The Shuttle used special windows that had to withstand (1) the extreme heat of re-entry, (2) the pressure difference between interior cabin pressure and the exterior vacuum of space, and (3) the temperature difference between the very low temperature outside and the shirt-sleeves temperature inside. The windows met these requirements by using three panes of fused quartz glass. The windows were also optically free of distortions so that astronauts could take photos through them. In this NASA image two astronauts taking a spacewalk look inside the Shuttle from outside.
 A thermos bottle is designed to minimize heat transfer. The goal is to keep the fluid inside hot – or cold – for as long as possible. Thermos bottles were cleverly designed to reduce conduction, convection and radiation. They are made of two glass flasks, one inside the other. A narrow space between them is partially evacuated of air, and both sides of the facing glass walls are silvered. The vacuum between the two flasks means that conduction and convection won’t operate because they require a material – such as air – to carry heat. By making the facing walls reflective like a mirror, radiation loss of heat is retarded between the two flasks. Some companies advertise that their thermos’ will keep coffee hot for up to 12 hours, but many user reviews say that newer ones made in China barely keep liquids warm for three hours. What changes do you think might have been made in the manufacture of thermos bottles that reduced their insulating ability?
A thermos bottle is designed to minimize heat transfer. The goal is to keep the fluid inside hot – or cold – for as long as possible. Thermos bottles were cleverly designed to reduce conduction, convection and radiation. They are made of two glass flasks, one inside the other. A narrow space between them is partially evacuated of air, and both sides of the facing glass walls are silvered. The vacuum between the two flasks means that conduction and convection won’t operate because they require a material – such as air – to carry heat. By making the facing walls reflective like a mirror, radiation loss of heat is retarded between the two flasks. Some companies advertise that their thermos’ will keep coffee hot for up to 12 hours, but many user reviews say that newer ones made in China barely keep liquids warm for three hours. What changes do you think might have been made in the manufacture of thermos bottles that reduced their insulating ability?
Heat Transfer and the Environment
 As discussed above, heat loss through windows is a problem. Keeping heat inside is good for the environment – because less energy is used - and it saves money too. Heat is lost from buildings not just through windows but everywhere, especially through roofs because heat rises in a house, making attics very hot. All homes have insulation (made with material of low thermal conductivity) inside their roofs to slow the loss of heat, but sometimes the insulation is not distributed everywhere equally. After a winter snowfall you can judge the adequacy of attic insulation by looking at roofs. If the snow covers all the roof the insulation is doing its job. But if there are places on the roof where the snow has melted then that is where the insulation is inadequate. Sealing air leaks and adding more insulation will reduce heating costs. Physics to the rescue!
As discussed above, heat loss through windows is a problem. Keeping heat inside is good for the environment – because less energy is used - and it saves money too. Heat is lost from buildings not just through windows but everywhere, especially through roofs because heat rises in a house, making attics very hot. All homes have insulation (made with material of low thermal conductivity) inside their roofs to slow the loss of heat, but sometimes the insulation is not distributed everywhere equally. After a winter snowfall you can judge the adequacy of attic insulation by looking at roofs. If the snow covers all the roof the insulation is doing its job. But if there are places on the roof where the snow has melted then that is where the insulation is inadequate. Sealing air leaks and adding more insulation will reduce heating costs. Physics to the rescue!
Image: © Ruslan Solntsev - Fotolia.com
© 2013 by Wheeling Jesuit University/Center for Educational Technologies®. 316 Washington Ave., Wheeling, WV 26003-6243. All rights reserved. Privacy Policy and Terms of Use.


